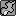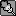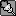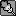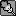THanks for showing to me man. Really appreciate it.
But could you find any nitpicks into how I did it? I don't understand how using the power rule, and using the chain rule screwed up =/
Man calculus is a bitch if you don't study. We started this a week and a half ago, and I just started now.
But could you find any nitpicks into how I did it? I don't understand how using the power rule, and using the chain rule screwed up =/
Man calculus is a bitch if you don't study. We started this a week and a half ago, and I just started now.